Journal Club: Jumping genes reveal birds and their sex chromosomes evolved together
SUMMARY: Avian retroposons — “jumping genes” — reveal that birds and their sex chromosomes evolved together, and provide us with important clues into the evolution of sex chromosomes and sex in general
![]() Like mammals, the sex of individual birds is determined by the combination of sex chromosomes they get from their parents at fertilization. But unlike mammals, where females are the homogametic sex possessing two copies of the same sex chromosome, males are the homogametic sex. This difference to the mammalian sex chromosome system is indicated by the name: instead of X and Y, avian sex chromosomes are known as Z and W. Similar to mammalian sex chromosomes, avian sex chromosomes consist of one large chromosome (Z) and one very small, degenerate chromosome (W), which evolved from a pair of autosomes (non-sex chromosomes). However as one might predict, Z and W arose independently and their evolution followed an independent trajectory from that of mammalian X and Y chromosomes.
Like mammals, the sex of individual birds is determined by the combination of sex chromosomes they get from their parents at fertilization. But unlike mammals, where females are the homogametic sex possessing two copies of the same sex chromosome, males are the homogametic sex. This difference to the mammalian sex chromosome system is indicated by the name: instead of X and Y, avian sex chromosomes are known as Z and W. Similar to mammalian sex chromosomes, avian sex chromosomes consist of one large chromosome (Z) and one very small, degenerate chromosome (W), which evolved from a pair of autosomes (non-sex chromosomes). However as one might predict, Z and W arose independently and their evolution followed an independent trajectory from that of mammalian X and Y chromosomes.
But within Aves, the sex chromosomes of different groups, or clades, of birds, are at different stages of evolution. For example, the neognaths, which include most of the birds that commonly visit your bird feeders, have ZW chromosomes that resemble mammalian XY chromosomes: one very large chromosome paired with one tiny, degenerate chromosome. On the other hand, the paleognaths, which include the ostriches and similar mostly terrestrial, flightless birds, have sex chromosomes that look like autosomes. In fact, their sex chromosomes behave a lot like autosomes, too; recombining along much of their length to swap genetic information (doi:10.1023/A:1009278914829). But that said, there is one paleognath lineage, the neotropical tinamous, that shows an intermediate level of Z-W differentiation (doi:10.1007/s00412-006-0088-y).
But have the genes located on the sex chromosomes evolved similarly to each other? And what does the pattern of sex chromosome evolution tell us about the evolution of birds?
You may recall that I recently wrote about retroposons, often referred to in the mainstream media as “jumping genes”.
Retroposons are repetitive DNA fragments that are sprinkled throughout the genome. They are copied (“reverse transcribed”) from an RNA intermediary that randomly “jumps” to another locus within the genome where they insert themselves. The original repetitive retroposon sequence identities and locations are inherited like other genetic loci, but the new insertion locations (along with any changes in sequence) are unique and are reliably inherited from the time they are inserted. The resulting patterns of change are easy to see and can be followed through ensuing generations for more than 100 million years — major evolutionary timescales. In short, retroposons are molecular fossils.
Like other chromosomes, sex chromosomes carry retroposons. Since similar genes (gametologues) on the avian Z and W chromosomes are not swapping genetic information, their evolution can be mapped by the presence or absence of retroposons (figure 1):
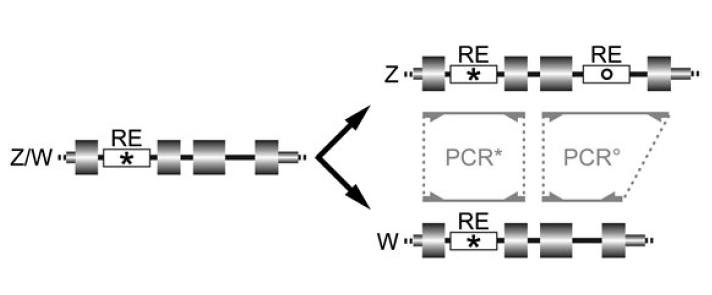
The above diagramme shows how a pair of gametologues change as retroposed elements (REs) insert into each member of the pair at different times. The large gray boxes are exons (genes), small gray boxes are untranslated regions (not encoding any proteins), and the white boxes are retroposons [figure 1, larger view].
The gene on the left represents the pair whilst they were crossing over frequently. At that point, they look the same. However, after recombination stops, these regions on the sister chromosomes are free to diverge independently along their own evolutionary pathways, giving rise to two very similar, but no longer identical, gametologues (right side). REs that inserted prior to the cessation of crossing over (asterix) are inherited by both gametologues, whilst those REs that “jumped” after crossing over ceased (circle) are unique to just one of the gametologues. Of course, the corresponding regions of the two gametologues are different lengths, too.
Alexander Suh, a graduate student who studies phylogenomics at Münster University in Germany, sees retroposons as an unique way to study the evolution of avian sex chromosomes. Mr Suh spearheaded the effort to screen all 12 gametologous genes known from the chicken genome. His team was specifically looking to identify Z-/W-presence or Z-presence/W-absence regions amongst the 126 sequenced intron pairs for all birds (doi:10.1534/genetics.108.090324). They identified four such regions in three gametologues: introns 9 and 16 of the chromodomain helicase DNA-binding protein 1 (CHD1) gene, intron 16 of the avian homologue to the Drosophila Nipped-B (NIPBL) gene, and intron 3 of the ATP synthase α-subunit isoform 1 (ATP5A1) gene. Analyses of RE insertion patterns for each locus yielded three phylogenetic trees (data for CHD1 introns 9 & 16 are combined into one tree) (figure 2):
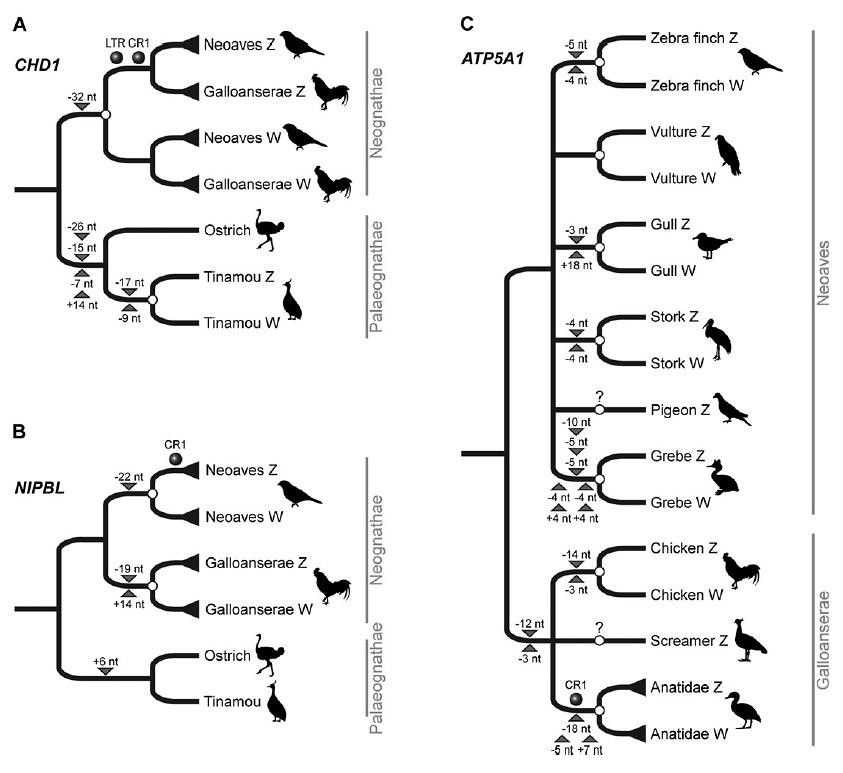
The overall shape of the CHD1 gene tree (figure 2a; larger view) corroborates previous research showing an independent differentiation of this gene pair in tinamous and in neognaths (doi:10.1007/s00412-006-0088-y). These RE insertion patterns were also confirmed with sequence analyses.
Not surprisingly, a different tree topology was obtained for NIPBL, because recombination ceased independently in this gene pair in the neoavian and in the galloanseran lineage (figure 2b; larger view). Unfortunately, it’s not clear from the DNA data whether recombination ceased in the ancestor of Galloanserae or shortly after they diverged.
The gene tree of ATP5A1 is quite complex. It looks as though recombination ceased independently in all six neoavian representatives that were included in this study (figure 2c; larger view), but more study and more avian representatives are necessary to better understand these patterns and when they occurred.
“We have looked at sex chromosome evolution in birds from a perspective based on rare genomic changes,” writes Mr Suh in email.
As I already mentioned, retroposon insertions are very useful for uncovering evolutionary relationships. But this new study shows yet another use of retroposons: helping us to understand the temporal sequence of sex chromosome evolution and gametolog differentiation. By tracing the pattern of RE insertions in gametologues of living bird species, it is possible to follow this back to the common ancestor of their sex chromosomes.
“To our knowledge, this has not been done in any other sex chromosomal system yet — so it should be promising to have a look at the many other sex chromosomal systems (e.g., in mammals, snakes, some turtles, some fish, some plants) from this perspective.”
Retroposons provide another source of molecular information that is independent of autosomal or mitochondrial DNA sequences. But taken together, the RE insertions and other rare genomic changes suggest that the CHD1Z/CHD1W genes were already differentiated in the common ancestor for neognaths (119-105 mya; doi:10.1534/genetics.108.090324). Additionally, the NIPBLZ/NIPBLW genes also diverged early, in the neoavian ancestor (105-97.3 mya), whereas the ATP5A1Z/ATP5A1W genes appear to have diverged fairly recently (53 mya) (doi:10.1534/genetics.108.090324).
“The process of sex chromosome evolution and the dynamics of their regional differentiation is a fascinating topic,” says Mr Suh. “Understanding the similarities and differences in the many independent evolutions of sex chromosomal systems promises to yield insights into general processes involved in the evolution of sex determination (and sex in general).”
Besides being fascinating research, the ornithologists, conservation biologists and aviculturists in the crowd have probably already noticed a practical application for this research: using the visible size difference of these REs to identify the sex of birds.
As we already saw, retroposon insertions increase the size of a particular region within a gametologue by several hundred basepairs. Female birds, having a Z and W chromosome, will carry two distinct copies of these gametologues, whilst males, being ZZ, have just one. Thus, when any of these four regions are copied a million-fold using PCR, then separated by size using gel electrophoresis, the resulting PCR amplicons provide an unambiguous and fast method for identifying sex of birds (eggs, feathers, etc.) from nearly all Neoavian or Neognathan species, depending upon the region amplified. Further, amplifying more than one region can provide an internal check against false results (figure 3):
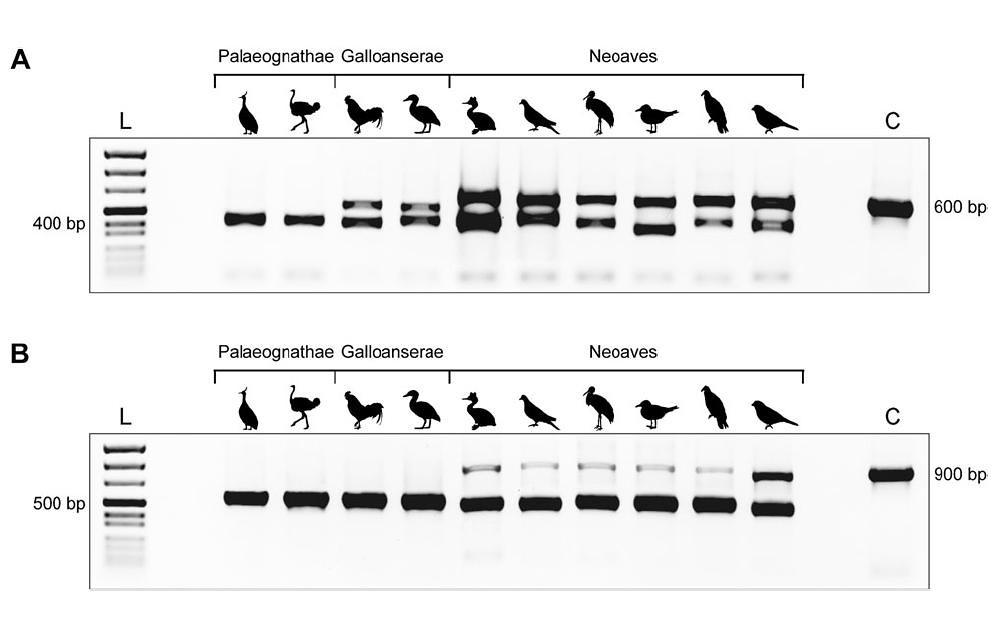
“[I]t’s nice to see that ‘by chance’ it’s possible to find something that is
of relevance to be patented and might be of use to ornithologists for
convenient bird sex identification,” said Mr Suh in email.
“The nice thing about these three sexing tests is that because we know when the retroposon inserted into each test locus, we know for which species the test should be (virtually) applicable. All three retroposon insertions are quite ancient, so now there are three independent sexing tests for almost all birds and with a distinct size difference between the female-specific W band and the Z band.”
NOTE: this piece is mirrored from here.
Sources:
Suh, A., Kriegs, J., Brosius, J., & Schmitz, J. (2011). Retroposon Insertions and the Chronology of Avian Sex Chromosome Evolution. Molecular Biology and Evolution DOI: 10.1093/molbev/msr147
Alex Suh [emails]
Some background reading on avian retroposons.
Further reading:
Kiwoong Nam and Hans Ellegren. (2008). The Chicken (Gallus gallus) Z Chromosome Contains at Least Three Nonlinear Evolutionary Strata. Genetics 180:1131-1136 doi:10.1534/genetics.108.090324
Akira Ogawa, Koichi Murata, and Shigeki Mizuno. (1998). The location of Z- and W-linked marker genes and sequence on the homomorphic sex chromosomes of the ostrich and the emu. PNAS 95(8):4415-4418. [abstract with link to free PDF]
Swathi Shetty, Darren K. Griffin and Jennifer A. Marshall Graves. (1999). Comparative Painting Reveals Strong Chromosome Homology Over 80 Million Years of Bird Evolution. Chromosome Research 7(4):289-295. doi:10.1023/A:1009278914829
.. .. .. .. .. .. .. .. .. .. .. ..
twitter: @GrrlScientist
facebook: grrlscientist
email: grrlscientist@gmail.com
Top Photo: Ostriches in Namibia by GeoftheRef on Flickr.
